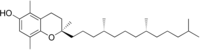
β-生育酚(英語:β-Tocopherol)是四種生育酚之一,分子式C28H48O2,是構成维生素E的化合物之一[2][3]。在氯化镁、三乙胺存在下,它和甲醛反应,得到羟基邻位甲酰化的产物;[4]它和四丁基三溴化铵反应,得到羟基邻位溴化的产物;[5]和溴-氢氧化钠反应时,则发生氧化-卤化和重排反应。[6]
- ^ "Drugs - Synonyms and Properties" data were obtained from Ashgate Publishing Co. (US). 2000. ISBN 0-566-08228-4. Retrieved from SciFinder. [2021-11-21].
- ^ Food and Agriculture Organization; World Health Organization. 9. Vitamin E. Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (报告). Bangkok, Thailand: FAO Rome. 2001 [2021-11-20]. (原始内容存档于2022-04-01).
- ^ Burton, G. W.; Ingold, K. U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. Journal of the American Chemical Society. 1981, 103 (21): 6472–6477. doi:10.1021/ja00411a035.
- ^ Khaled Alsabil, Guillaume Viault, Sorphon Suor-Cherer, Jean-Jacques Helesbeux, Joumaa Merza, Vincent Dumontet, Luis Manuel Peña-Rodriguez, Pascal Richomme, Denis Séraphin. Efficient ortho-formylation in vitamin E series, application to the semi-synthesis of natural 5- and 7-formyl-δ-tocotrienols revealing an unprecedented 5-bromo-7-formyl exchange. Tetrahedron. 2017-12, 73 (49): 6863–6870 [2021-11-20]. doi:10.1016/j.tet.2017.10.039. (原始内容存档于2018-06-15) (英语).
- ^ Jia-fei Poon, Vijay P. Singh, Jiajie Yan, Lars Engman. Regenerable Antioxidants-Introduction of Chalcogen Substituents into Tocopherols. Chemistry - A European Journal. 2015-02-02, 21 (6): 2447–2457 [2021-11-20]. doi:10.1002/chem.201405895 (英语).
- ^ Stefan Böhmdorfer, Anjan Patel, Andreas Hofinger, Thomas Netscher, Lars Gille, Thomas Rosenau. Bromination of Tocopherols: Oxidative Halogenations and Rearrangements. European Journal of Organic Chemistry. 2011-06, 2011 (16): 3036–3049 [2021-11-20]. doi:10.1002/ejoc.201100153 (英语).


 French
French Deutsch
Deutsch