Nitrate - Simple English Wikipedia, the free encyclopedia
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name Nitrate | |||
| Identifiers | |||
CompTox Dashboard (EPA) | |||
| Properties | |||
| NO− 3 | |||
| Molar mass | 62.0049 g mol-1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
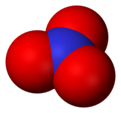
Nitrate (NO31-) is a polyatomic ion. It is made up of one nitrogen and three oxygen atoms. It is part of many important molecules. Potassium nitrate is a common type of nitrate. It is often used in fertilizers because plants and crops need both potassium and nitrates to live and grow. Sodium nitrate is also used in preserving foods.[1]
Some nitrates are explosive. People make large amounts of nitrates from ammonia. Nitrates are similar to nitrites. Many metal nitrates with thermal decomposition makes oxygen and metal nitrate.
Nitric acid has the formula HNO3 and has no overall charge, because the hydrogen ion is positive.
Nitrate has a group valency of 1.
References[change | change source]
- ↑ "The unhealthy preservative hiding in processed meats". Mayo Clinic. Retrieved 2020-10-08.


 French
French Deutsch
Deutsch