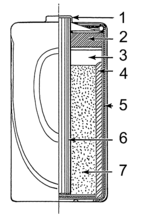
Electric battery (redirect from Wet cell)
Other primary wet cells are the Leclanche cell, Grove cell, Bunsen cell, Chromic acid cell, Clark cell, and Weston cell. The Leclanche cell chemistry was...
68 KB (7,300 words) - 21:41, 15 May 2024
A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry...
7 KB (835 words) - 17:17, 23 March 2024
Nickel–cadmium battery (redirect from Wet NiCD)
although this brand name is commonly used to describe all Ni–Cd batteries. Wet-cell nickel–cadmium batteries were invented in 1899. A Ni–Cd battery has a terminal...
33 KB (4,831 words) - 03:53, 21 April 2024
History of the battery (redirect from Wet battery)
"Wet cells" were open containers that held liquid electrolyte and metallic electrodes. When the electrodes were completely consumed, the wet cell was...
35 KB (4,480 words) - 02:29, 10 May 2024
Rice (redirect from Drying a wet cell phone in rice)
tropical crop, it can be grown during the two distinct seasons (dry and wet) of the year provided that sufficient water is made available. It is normally...
76 KB (7,370 words) - 09:16, 14 May 2024
Lead-acid battery (redirect from Lead-acid cell)
known as "gassing". Wet cells have open vents to release any gas produced, and VRLA batteries rely on valves fitted to each cell. Catalytic caps are available...
58 KB (7,490 words) - 18:54, 13 May 2024
VRLA battery (redirect from Gel cell)
problems) common to the wet cell battery, and boast greater resistance to shock and vibration. Chemically they are almost the same as wet (non sealed) batteries...
25 KB (3,203 words) - 02:56, 18 April 2024
"Agglomerate block cell" and the "Sack cell". Leclanché first, and Carl Gassner later, both strived to transform the original wet cell into a more portable...
10 KB (1,265 words) - 19:51, 12 February 2024
List of battery types (section Battery cell types)
more electrochemical cells. Three lists are provided in the table. The primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery...
6 KB (203 words) - 04:26, 1 April 2024
reaction in either half-cell). In the above wet-cell during discharge, nitrate anions in the salt bridge move into the zinc half-cell in order to balance...
16 KB (2,117 words) - 21:50, 7 May 2024