 | thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change ΔH⚬ is negative." Exothermic reactions usually release... 5 KB (552 words) - 07:35, 19 March 2024 |
Endothermic process (redirect from Endothermic reaction) "endothermic" is used to describe a reaction where energy is taken "(with)in" by the system (vs. an "exothermic" reaction, which releases energy "outwards")... 7 KB (809 words) - 18:40, 20 November 2023 |
Van 't Hoff equation (section Exothermic reactions) For an exothermic reaction ΔrH < 0, so slope=−ΔrHR>0.{\displaystyle {\text{slope}}=-{\frac {\Delta _{r}H}{R}}>0.} Thus, for an exothermic reaction, the... 22 KB (2,893 words) - 17:49, 30 December 2023 |
 | all living organisms. Exergonic Exergonic reaction Exothermic Endothermic Exothermic reaction Endothermic reaction Endotherm Exotherm Portal: Chemistry... 7 KB (938 words) - 07:14, 24 October 2023 |
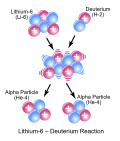 | etc. The reaction above would be written as 6Li(d,α)α. Kinetic energy may be released during the course of a reaction (exothermic reaction) or kinetic... 20 KB (2,392 words) - 20:03, 20 April 2024 |
Outline of chemistry (section Exothermic Reactions) that can describe the characteristics of a chemical reaction. Exothermic –a process or reaction in which the system release energy to its surroundings... 33 KB (4,048 words) - 23:47, 7 February 2024 |



