 | A data monitoring committee (DMC) – sometimes called a data and safety monitoring board (DSMB) – is an independent group of experts who monitor patient... 5 KB (679 words) - 22:58, 29 February 2024 |
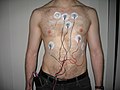 | Cardiac monitoring generally refers to continuous or intermittent monitoring of heart activity to assess a patient's condition relative to their cardiac... 12 KB (1,325 words) - 09:29, 9 March 2024 |
Institutional review board (redirect from Institutional Ethics Committee) Clinical trial Data monitoring committee Declaration of Helsinki Ethical problems using children in clinical trials Ethics committee (European Union)... 37 KB (4,462 words) - 16:19, 16 February 2024 |
mesh-based intercom system developed for motorcycle communication Data monitoring committee for clinical trials Dimethyl carbonate, a chemical compound Double... 3 KB (341 words) - 11:29, 10 February 2024 |
Data monitoring committees (EMA) deals with independent data monitoring committees. It highlights the key issues involved when sponsors include data monitoring... 29 KB (3,027 words) - 20:22, 9 February 2024 |
 | However, the trial was terminated in February 2018, after a data monitoring committee concluded it was unlikely that the drug would show a positive... 8 KB (575 words) - 18:14, 5 December 2023 |
system by Red Digital Cinema Camera Company Data and Safety Monitoring Committee, see Data monitoring committee D.S. Senanayake College Media circle, see... 887 bytes (140 words) - 15:21, 26 January 2024 |
 | Self-Monitoring, Analysis, and Reporting Technology (S.M.A.R.T., often written as SMART) is a monitoring system included in computer hard disk drives... 82 KB (5,331 words) - 15:25, 23 April 2024 |
(WHO), require data and safety monitoring protocols for Phase I and II clinical trials conforming to their standards. Safety monitoring of a clinical trial... 7 KB (808 words) - 01:54, 9 December 2023 |

