AM-1221
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
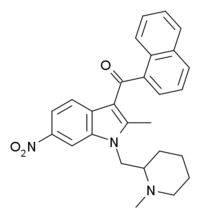
| Formula | C27H27N3O3 |
| Molar mass | 441.531 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2.[1] The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.[2][3]
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-1221 are Schedule I Controlled Substances.[4]
Legal status
[edit]It is illegal to supply, trade, sell, distribute, import or transport the pharmaceutical drug in the UK under the Psychoactive Substances Act 2016 which was in force on May 26, 2016.
See also
[edit]References
[edit]- ^ WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ^ Deng H (2000). Design and synthesis of selective cannabinoid receptor ligands: Aminoalkylindole and other heterocyclic analogs (Ph.D. Dissertation). University of Connecticut. ProQuest 304624325.
- ^ Manera C, Tuccinardi T, Martinelli A (April 2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- ^ : Schedules of controlled substances


 French
French Deutsch
Deutsch