bromine (Br2) and iodine (I2) also form diatomic gases. All halogens have been observed as diatomic molecules, except for astatine and tennessine, which...
21 KB (2,550 words) - 01:42, 18 March 2024
symmetry group of the molecule. Among all the molecular symmetries, diatomic molecules show some distinct features and they are relatively easier to analyze...
60 KB (7,796 words) - 21:29, 29 June 2023
homonuclear molecules are diatomic molecule, which consist of two atoms, although not all diatomic molecules are homonuclear. Homonuclear diatomic molecules include...
2 KB (233 words) - 02:42, 28 April 2024
carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. The electrons in diatomic carbon are distributed among...
14 KB (1,124 words) - 17:54, 18 March 2024
f = 7 degrees of freedom, the maximum for a diatomic molecule. At high enough temperatures, all diatomic gases approach this value. Quantum mechanics...
50 KB (5,695 words) - 13:51, 6 October 2023
either the same lower level or the same upper level. For example, in a diatomic molecule the line denoted P(J + 1) is due to the transition (v = 0, J + 1)...
49 KB (6,784 words) - 20:47, 30 April 2024
Molecular vibration (redirect from Vibrating molecule)
300 to 3000 cm−1 and wavelengths of approximately 30 to 3 μm. For a diatomic molecule A−B, the vibrational frequency in s−1 is given by ν = 1 2 π k / μ...
20 KB (2,706 words) - 17:20, 16 May 2024
Molecular orbital diagram (redirect from Diatomic molecular orbital diagram)
paramagnetic diatomic. The bond order of diatomic oxygen is two. MO treatment of dioxygen is different from that of the previous diatomic molecules because...
38 KB (4,966 words) - 02:30, 13 March 2024
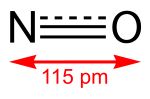
Nitric oxide (category Diatomic molecules)
formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding...
29 KB (2,754 words) - 05:37, 31 May 2024
Chemical polarity (redirect from Polar molecule)
208 Å. A useful conversion factor is 1 D = 3.335 64×10−30 C m. For diatomic molecules there is only one (single or multiple) bond so the bond dipole moment...
24 KB (2,750 words) - 14:34, 8 June 2024